ABOUT USNeuroActiva, Inc. is a wholly owned subsidiary of Biomed Industries, Inc, which is also parent company of Biomed Pharmaceuticals, Inc, Biomed AI and BiopharmaRX. BIOMED PHARMACEUTICAL Biomed Pharmaceutical was a pioneer in developing new anti-malarial and antiviral drugs dated back in 1990s. During that time, there were more 100 million cases of malaria and 1 million people mostly children died in Africa and Asia. The company developed a new drug called Artemether, which has been demonstrated as an effective drug to treat Plasmodium falciparum malaria, that are resistant to chloroquine, which was the standard treatment for malaria at the time. Artemether has been approved as the last defense drug against chloroquine resistant Plasmodium falciparum malaria in more than 80 countries, include the FDA in the USA. Artemether/Lumefantrine is presently distributed worldwide by Novartis International AG. With more than 25 year experience in drug development, Biomed has focused its resources to developing new drugs for the prevention and treatment of Covid-19. NEUROACTIVA, INC. At the present time, Alzheimer's and Covid-19 are two of the most dreadful and deadly diseases that can not be prevented, stopped or cured. The current unmet needs represent a huge opportunity for pharmaceutical companies, which are developing targeted novel therapies. ALZHEIMER'S DISEASE Almost all Alzheimer drug candidates failed in Phase 3 over the past 20 years. These drugs were developed based on the guiding principle of the Amyloid hypothesis. The Amyloid Hypothesis proposed in 1984 postulates that accumulation of amyloid plaques triggers a cascade that harms neurons and synapses. The common approach over the past 20 years has been to use immunotherapy or modulation of the γ-secretase , β-secretase cleavage enzyme BACE1 to remove amyloid plaques. We believe that the amyloid hypothesis is wrong, and we need to find a new pathway. NEUROACTIVA’S SOLUTIONS:NeuroActiva™ has developed a new platform of new drug candidates for treatment of Alzheimer’s and other neurological diseases. Our guiding principle is based on proven clinical strategies of: Traneurocin ™ (NA-831™) NeuroActiva™ has developed a new platform of new drug candidates for treatment of Alzheimer’s and other neurological diseases with innovative mechanism of action. Our flagship drug is called Traneurocin™ or NA-831™ The drug NA-831™ is a small molecule drug that can be administered orally. The drug is an endogenous compound i.e. it already exists in the human brain. Basically, the drug is very safe and has no toxicity, suitable for long term treatment and prevention purpose. Neuroactiva plans to conduct two Phase 3 programs for NA-831: (1) The COGNITION Program for the treatment of 375 patients with mild and moderate Alzheimer’s disease over 12 months. Age group: 65- 80 years of age. (2) The PREVENTION Program for the prevention of 550 subjects, asymptomatic from a high risk population over 24 months. Age group: 45-70 years of age. Our drug treatment strategy is to develop neuroprotective interventions for the severe acute respiratory syndrome due to Covid-19 infections and provide appropriate rehabilitation measures afterwards. BIOCOVAX™ Biomed has developed Biovaccine™, which is comprised of an oral polio vaccine with our neuroprotective agent called NA-831. The oral polio vaccine has been approved by the FDA and widely used in the US until 2004 when the polio was declared eradicated in the country. BioCovax™ is an oral formulation which can be used as a prophylaxis and treatment of early onset of Covid-19. Biomed plans to launch the Phase 3 trials of BioCovax™ in early autumn 2020. BIOMEDIVIR™ Biomed has developed Biomedivir™, which is comprised of our neuroprotective agent called NA-831 and Atazanavir. Atazanavir was approved by the FDA for prevention and treatment of HIV/AIDS treatment, and is now a generic drug. Biomedivir™ is an oral formulation which can be used as treatment for mild and moderate of Covid-19 infections. Biomed plans to launch the Phase 2/3 trials of Biomedivir™ in early autumn 2020. NANOMEDIVIR™ Biomed has developed Nanomedivir™, which is comprised of NA-831 and Remdesivir, anti-viral drug in nanoparticle-based intranasal formulation. Remdesivir (made by Gilead Sciences has been approved by the FDA for emergency use of severe cases of Covid-19. Biomed plan to launch the Phase 1 clinical trials of Nanomedivir™ in autumn 2020. DEXANEUROSONE™ In addition, Biomed has also developed Dexaneurosone™, which is comprised of NA-831 and Dexamethasone in both oral and intranasal formulation. Dexamethasone is an FDA anti-inflammatory drug has been on the market for some 60 years and is used to treat conditions including arthritis and severe asthma. DDexaneurosone™ could be used to treat early and moderate cases of Covid-19. Biomed plan to launch the Phase 2/3 clinical trials of Dexaneurosone™ in autumn 2020. NOTE: NA-831 (Traneurocin), BioCovax, Biomedivir, Nanomedivir and Dexaneurosone are experimental drugs in clinical evaluation, and are not available to patients in the USA, until we receive the FDA approval. Patients should consult with physicians about the drugs. For further information, please contact us by completing Contact an Online Contact Form. Thank you. |
Prophylaxis and treatment for Alzheimer's disease and Covid-19:
-
Covid-19 Neuro-invasion
SARS-CoV-2 can directly invade the CNS. NeuroActiva has developed neuroprotective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards.
-
NA-831 (Traneurocin)
(NA-831™)(Traneurocin™) is a novel neuroprotective and neurogenesis drug for treatment of Alzheimer's disease which will be in Phase 3 in 2020-2021
-
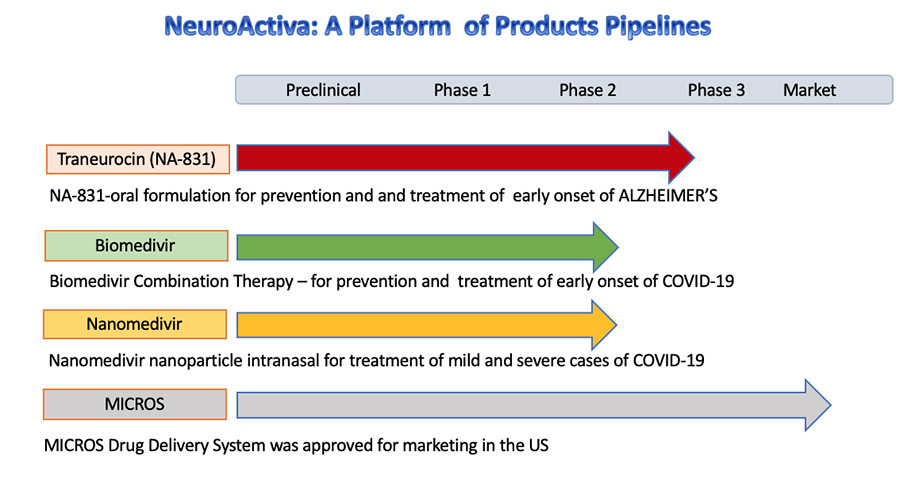
NA-831™ Drug Therapy
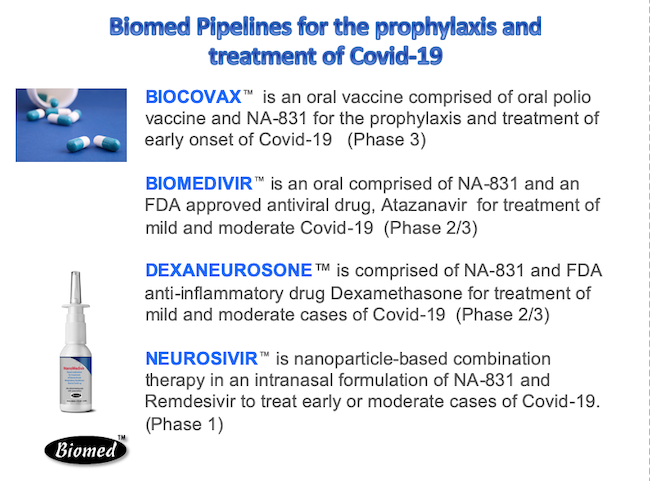
We have developed drug combination therapy known as Biomedivr™, Neuromedivir™, Dexaneurosone™ Neurosivir™ for the prophylaxis and treatment of Covid-19
-
MICROS™ Infusion System
The MICROS is a FDA approved controlled release infusion system for intravenous administration of injectable drugs, including NA-704 for treatment of neurological diseases.