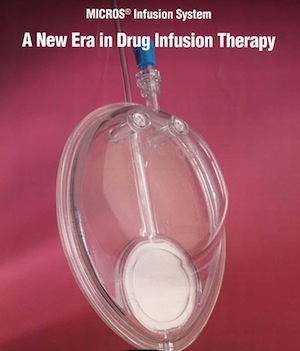
MICROS™ INFUSION SYSTEMS
The MICROS Infusion System is an intravenous drug delivery system that is capable of control release of more than 100 injectable drugs for treatment of infectious diseases, cancer chemotherapy and cardiovascular diseases.
The MICROS™ is made of a patented nano-biomembrane controlled release system that can be customized for the IV administration of NA-704 for treatment of neurological diseases.
The MICROS™ Infusion System has been approved by the United Stated Food and Drug Administration (FDA) for marking in the USA.
MICROS™ Infusion System Diagram

* An advanced infusion system for more than 100 injectable drug formulations for treatment of infectious diseases, cancer chemotherapy, cardiovascular and neurological diseases.
* Nano-biomembrane offers a high efficiency and greay capacity of drug loading-up to 98%.
* Nano-biomembrame (0.1 to 0.2 micron) ensure aseptic system eliminating particulates, bacteria, and endotoxin.
* The MICROS™ can be prefilled with a customized dosage of NA-704 to be available as a premixed and ready to for Alzheimer patients at home
NeuroActiva™ focuses on developing of treatment of Alzheimer's disease:
-

Alzheimer's Disease
Alzheimer's disease is the only cause of death in the top 10 in America that cannot be prevented, cured or slowed. Alzheimer is the 6th leading cause of death in the USA.
-
Research
NeuroActiva research focuses on the neuroprotection and regeneration of cells and tissue with its ability to repair damage caused by disease, trauma or age.

Clinical
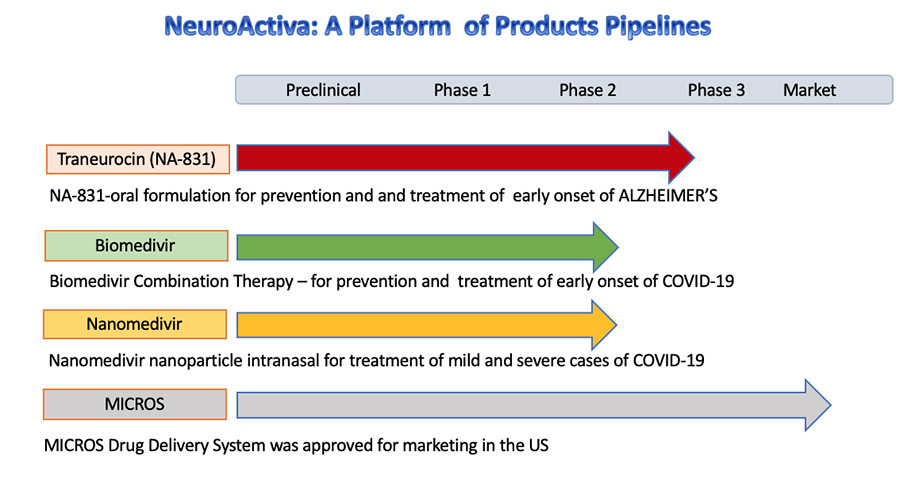
Our new platform of neuroprotective and neurogenesis drugs for treatment of Alzheimer including Traneurocin™ (NA-831™) which will be in Phase 3 clinical trials on Alzheimer patients in 2020.
-
Vineurocin™ and Traneurocin™
Vineurocin and Traneurocin protect damaged neurons and stimulates regeneration of neurons, outgrowth of electrically active fibres, restoration of neuronal function, a major clinical feature of both Alzheimer's disease.
-
MICROS™ Infusion System
The MICROS is a FDA approved disposable infusion system for intravenous administration of injectable drugs, including Traneurocine for treatment of neurological diseases.